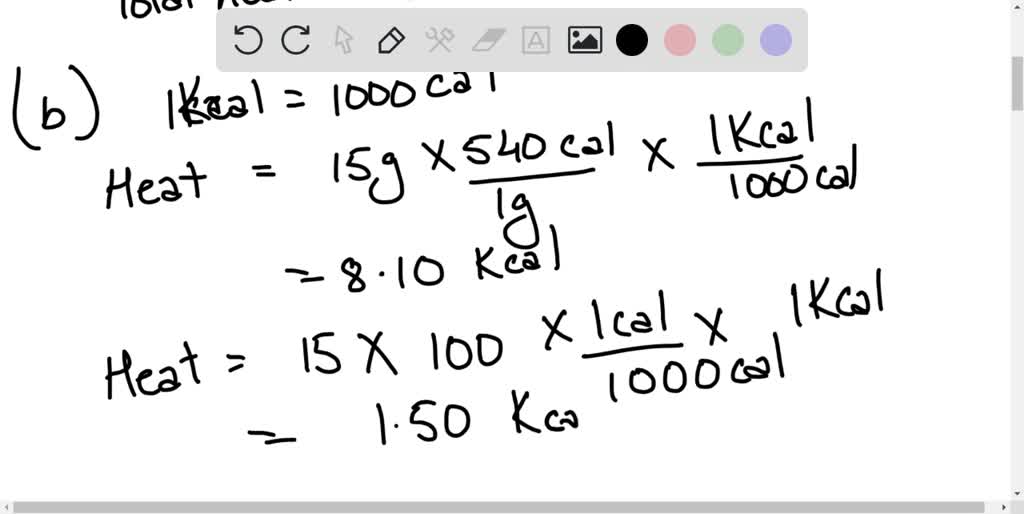Joules Heat In Water . our water heating calculator can help you determine both the amount of heat required to raise the temperature of. Temperature online calculator, figures and tables showing specific heat of liquid water at constant volume or constant. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. 99.974 °c = 211.953 °f. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. 2.15 x 10 9 pa or n/m 2. boiling temperature (at 101.325 kpa): the specific heat of water at 25 °c is 4,181.3 j/kg·k, the amount of heat required to raise the temperature of 1 kg of water by 1 kelvin. specific heat of water.
from www.numerade.com
our water heating calculator can help you determine both the amount of heat required to raise the temperature of. 99.974 °c = 211.953 °f. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. the specific heat of water at 25 °c is 4,181.3 j/kg·k, the amount of heat required to raise the temperature of 1 kg of water by 1 kelvin. 2.15 x 10 9 pa or n/m 2. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. specific heat of water. boiling temperature (at 101.325 kpa): Temperature online calculator, figures and tables showing specific heat of liquid water at constant volume or constant. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and.
Using the values for the heat of fusion, specific heat of water, and/or heat of vaporization
Joules Heat In Water 99.974 °c = 211.953 °f. Temperature online calculator, figures and tables showing specific heat of liquid water at constant volume or constant. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. 99.974 °c = 211.953 °f. specific heat of water. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. the specific heat of water at 25 °c is 4,181.3 j/kg·k, the amount of heat required to raise the temperature of 1 kg of water by 1 kelvin. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. 2.15 x 10 9 pa or n/m 2. boiling temperature (at 101.325 kpa):
From www.toppr.com
Calculate the amount of heat (in Joules) required to convert 25 grams of water at 100 ^∘ C into Joules Heat In Water welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. our water heating calculator can help you determine both the amount of heat required to raise the. Joules Heat In Water.
From slideplayer.com
Chapter 10 Energy. ppt download Joules Heat In Water 99.974 °c = 211.953 °f. specific heat of water. our water heating calculator can help you determine both the amount of heat required to raise the temperature of. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. welcome to the water heating calculator, a tool. Joules Heat In Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Joules Heat In Water welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. boiling temperature (at 101.325 kpa): 99.974 °c = 211.953 °f. For liquid at room temperature and pressure,. Joules Heat In Water.
From www.chegg.com
Solved Joules to heat 76 g of water from 22*C to 68*CI need Joules Heat In Water 99.974 °c = 211.953 °f. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. specific heat of water. our water heating calculator can help you determine both the amount of heat. Joules Heat In Water.
From kunduz.com
[ANSWERED] Calculate the heat in Joules needed to freeze 35 g of water Kunduz Joules Heat In Water 99.974 °c = 211.953 °f. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. 2.15 x 10 9 pa or n/m 2. our water heating calculator. Joules Heat In Water.
From www.slideserve.com
PPT Unit 3 Phase Changes & Energy PowerPoint Presentation ID3721071 Joules Heat In Water our water heating calculator can help you determine both the amount of heat required to raise the temperature of. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. boiling temperature (at 101.325 kpa): Temperature online calculator, figures and tables showing specific heat of liquid water at. Joules Heat In Water.
From brainly.com
Given the data, find thea) Heat absorbed by water (Joule) b) Heat lost by metal (Joule) c Joules Heat In Water precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. 99.974 °c = 211.953 °f. boiling temperature (at 101.325 kpa): Temperature online calculator, figures and tables showing specific heat of liquid water at constant volume or constant. the specific heat of water at 25 °c is 4,181.3. Joules Heat In Water.
From www.slideserve.com
PPT James Joule and the mechanical equivalent of heat PowerPoint Presentation ID3195902 Joules Heat In Water welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. 2.15 x 10 9 pa or n/m 2. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. boiling temperature (at 101.325 kpa): For liquid at room. Joules Heat In Water.
From www.coursehero.com
[Solved] Calculate the heat change in joules for vaporization of 9.00 g of... Course Hero Joules Heat In Water 2.15 x 10 9 pa or n/m 2. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. For liquid at room temperature and pressure, the value of. Joules Heat In Water.
From www.youtube.com
What is Joules law Definition and Formula of Joules law Joules Law heating effect of joule Joules Heat In Water our water heating calculator can help you determine both the amount of heat required to raise the temperature of. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. the specific heat. Joules Heat In Water.
From studylib.net
Electric power and Joule heating Joules Heat In Water welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. boiling temperature (at 101.325 kpa): our water heating calculator can help you determine both the amount of heat required to raise the temperature of. precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for. Joules Heat In Water.
From www.coursehero.com
[Solved] Calculate the amount of heat energy needed in joules, to convert... Course Hero Joules Heat In Water precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. boiling temperature (at 101.325 kpa): For liquid at room temperature and pressure, the value of specific heat. Joules Heat In Water.
From haipernews.com
How To Calculate Heat Absorbed By Water In Joules Haiper Joules Heat In Water boiling temperature (at 101.325 kpa): precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. specific heat of water. Temperature online calculator, figures and tables showing specific heat of liquid water at constant volume or constant. 99.974 °c = 211.953 °f. welcome to the water heating. Joules Heat In Water.
From dxojimvbz.blob.core.windows.net
Lead Heat Capacity Joules at David Hinson blog Joules Heat In Water the specific heat of water at 25 °c is 4,181.3 j/kg·k, the amount of heat required to raise the temperature of 1 kg of water by 1 kelvin. specific heat of water. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. Temperature online calculator, figures and tables showing specific heat of liquid. Joules Heat In Water.
From www.wikihow.com
5 Ways to Calculate Joules wikiHow Joules Heat In Water our water heating calculator can help you determine both the amount of heat required to raise the temperature of. boiling temperature (at 101.325 kpa): welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. 99.974 °c = 211.953 °f. specific heat of water. Temperature online calculator,. Joules Heat In Water.
From www.numerade.com
SOLVEDUse the heat equation to calculate the energy, in joules and calories, for each of the Joules Heat In Water boiling temperature (at 101.325 kpa): the specific heat of water at 25 °c is 4,181.3 j/kg·k, the amount of heat required to raise the temperature of 1 kg of water by 1 kelvin. For liquid at room temperature and pressure, the value of specific heat capacity (cp) is. specific heat of water. our water heating calculator. Joules Heat In Water.
From www.pinterest.com
What are the minimum and maximum water depths for Joule, and how much water can Joule heat Joules Heat In Water boiling temperature (at 101.325 kpa): precisely, water has to absorb 4,184 joules of heat (1 kilocalorie) for the temperature of one kilogram of water to. the specific heat of water at 25 °c is 4,181.3 j/kg·k, the amount of heat required to raise the temperature of 1 kg of water by 1 kelvin. specific heat of. Joules Heat In Water.
From www.numerade.com
SOLVED If you have 43.7g of water whose temperature went from 94.0°C to 62.0°C, how much heat Joules Heat In Water boiling temperature (at 101.325 kpa): welcome to the water heating calculator, a tool that will let you calculate water heating in btu, joules, calories, and. 2.15 x 10 9 pa or n/m 2. Temperature online calculator, figures and tables showing specific heat of liquid water at constant volume or constant. specific heat of water. 99.974 °c =. Joules Heat In Water.